Intelligent Innovation: Pioneering with Purpose
At the heart of our philosophy lies Intelligent Innovation, a guiding principle that transcends traditional invention. It’s about crafting solutions with a deeper understanding, insightful analysis, and the foresight to address the complexities of tomorrow. Our approach is meticulous and thoughtful, ensuring that every product, service, or process we introduce is not only novel but also rooted in intelligent design and sustainable practices.
Intelligent Innovation is more than just creating; it’s about solving problems in a way that is both smart and responsible. We leverage cutting-edge technology, comprehensive research, and collaborative thinking to develop solutions that are adaptable, user-centric, and impactful. Our commitment is to advance progress responsibly, ensuring that our innovations not just meet the current needs but also enrich lives and foster sustainability for future generations.
Product Pipeline
BioMed is enhancing its suite of offerings through Intelligent Innovation, developing specialized solutions for otolaryngology. Our deep expertise and dedication to innovation empower us to craft surgical products that excel in quality and performance. The growth of our portfolio reflects the trust our community has in us—a trust we’re committed to upholding with every innovation. We’re currently focused on developing the following products, each a testament to our commitment to intelligent, forward-thinking solutions:
Anti-Adhesive Gel for FESS
Functional Endoscopic Sinus Surgery (FESS) represents a significant advancement in treating sinus conditions, yet the post-surgical formation of scar tissue remains a common challenge that can impact recovery and outcomes. Leveraging our deep expertise in biopolymers and their regenerative properties, BioMed is at the forefront of addressing this issue with an innovative solution: a biodegradable hydrogel specifically designed for FESS applications.
Our hydrogel is engineered to be easily injectable, simplifying the application process during or after surgery. Its unique formulation targets the reduction of the inflammatory response commonly observed around the surgical site. By doing so, it plays a crucial role in minimizing the likelihood of scar tissue formation, a key factor in ensuring a smoother recovery and enhancing the overall effectiveness of the FESS procedure.
This biodegradable hydrogel is a testament to BioMed’s commitment to ‘Intelligent Innovation’ in medical technology. It not only represents a significant leap forward in post-surgical care for FESS patients but also underscores our dedication to developing products that offer superior characteristics and outcomes. With BioMed’s hydrogel, patients and healthcare professionals alike can look forward to a future where FESS recovery is markedly improved, underscored by reduced complications and optimized healing processes.
Slow-Release Steroid Hydrogel
At the heart of BioMed’s latest innovation lies our proprietary Core Technology, a breakthrough in medical science enabling the development of an advanced hydrogel designed for precision healing. This cutting-edge hydrogel is crafted using our unique process to create spherical particles, meticulously loaded with steroids for targeted therapeutic action.
The brilliance of BioMed Core Technology lies in its foundation of biopolymers. These biopolymers are specifically chosen for their biodegradable properties, ensuring that the hydrogel not only provides effective treatment but also harmonizes with the body’s natural processes. By finely tuning the degradation rate of these biopolymers, we achieve unparalleled control over the steroid release profile. This controlled release mechanism ensures a sustained therapeutic effect, delivering the medication exactly where and when it’s needed, thereby enhancing the healing process.
The advantages of this innovative approach are manifold. Patients benefit from a more efficient and targeted treatment with minimal systemic side effects, thanks to the localized delivery of steroids. Additionally, the biodegradable nature of the hydrogel aligns with our commitment to safety and environmental responsibility, ensuring that the product naturally integrates and then harmlessly dissolves within the body.
BioMed’s advanced hydrogel exemplifies our dedication to ‘Intelligent Innovation,’ offering a sophisticated solution that sets a new standard for medical treatments. It’s not just about healing; it’s about advancing the way we heal, ensuring every patient receives the most effective, precise, and compassionate care possible.
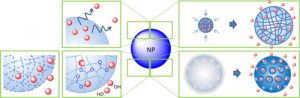
Controlled release of active in biopolymer construct
Technologies
BioMed ENT harnesses its profound knowledge and extensive experience with biopolymers to masterfully tune their characteristics, targeting the creation of straightforward solutions for complex challenges in otolaryngology. Through strategic collaborations with leading universities and hospitals, we continuously enhance our expertise, fostering the creation of new knowledge and innovative approaches. Our commitment to research and development has led to the creation and refinement of technologies that position us at the forefront of medical innovation, allowing us to curate the most advanced portfolio in the field. Dedicated to pushing the boundaries of what’s possible, we are constantly expanding our technological capabilities to deliver the finest solutions to our partners and patients.
BioMed Core Technology
BioMed ENT proudly introduces a groundbreaking patented platform technology, a cornerstone innovation that allows for the precise manufacturing of uniform spherical submicron particles from a diverse range of biopolymers. This cutting-edge process grants us unparalleled control over the particle formation, enabling us to finely tune the biopolymers’ characteristics, including degradation rates, absorption properties, and handling capabilities. Our technology stands as a testament to our commitment to advancing medical science, offering customizable solutions that meet the specific needs of our partners and enhance patient care.
Source Material

BioMed Core Technology

Spherical Submicron Particles